Research
We study neutrophil migration and the inflammation process using the zebrafish model. We are also interested in establishing novel infection models using zebrafish where pathogen-host interactions are made transparent. Currently we have 3 major projects in the lab.
Project #1: Identification and characterization of micro-RNAs that moderate acute neutrophilic inflammation
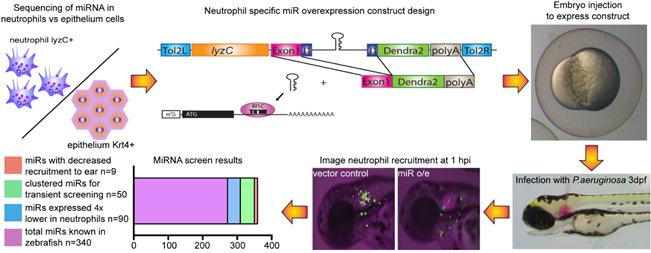
Using next generation sequencing, we have identified miRNAs that are enriched in neutrophils. We have finished a targeted screen and identified miRNAs that suppress neutrophil migration. We are currently characterizing the contribution of each individual miRNA in regulating neutrophil motility and their impact on the outcome during infections.
Project #2: MiR-223 and neutrophilic inflammation
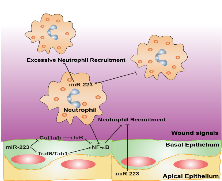 MicroRNA-223 is known as a myeloid-enriched anti-inflammatory microRNA that is dysregulated in numerous inflammatory conditions. We find that neutrophilic inflammation (wound response) is augmented in miR-223-deficient zebrafish, due primarily to elevated activation of the canonical NF-κB pathway in epithelial cells, and this direct connection between miR-223 and the canonical NF-κB pathway provides a mechanistic understanding of the multifaceted role of miR-223 and highlights an overlooked relevance of epithelial cells in dampening neutrophil activation.
MicroRNA-223 is known as a myeloid-enriched anti-inflammatory microRNA that is dysregulated in numerous inflammatory conditions. We find that neutrophilic inflammation (wound response) is augmented in miR-223-deficient zebrafish, due primarily to elevated activation of the canonical NF-κB pathway in epithelial cells, and this direct connection between miR-223 and the canonical NF-κB pathway provides a mechanistic understanding of the multifaceted role of miR-223 and highlights an overlooked relevance of epithelial cells in dampening neutrophil activation.
Project #3: Mitochondria and neutrophil migration
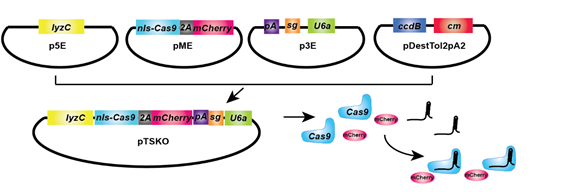
Neutrophils are fast moving cells essential for host immune functions, and they primarily rely on glycolysis for ATP. Whether mitochondria regulate neutrophil motility in vivo, however, and the underlying molecular mechanisms remain obscure. To dissect mitochondrial function genetically, we establish a system harboring the CRISPR/Cas9 elements for tissue-specific knockout. With this system, we provide the first in vivo evidence that mitochondria regulate neutrophil motility, tools for the functional characterization of mitochondria related genes in neutrophils, and insights into immune deficiency seen in patients with primary mitochondrial disorders.
