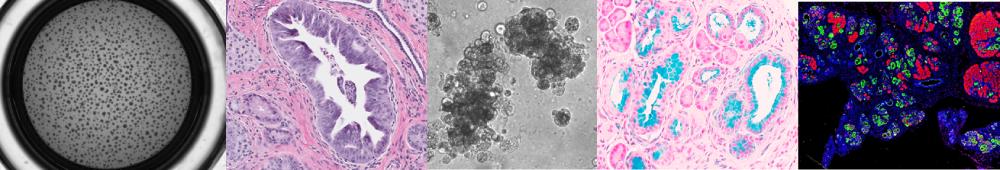
Research
During oncogenesis, kinases are constitutively activated, and phosphatases, such as Protein Phosphatase 2A (PP2A), are suppressed leading to aberrant activation of signaling pathways that regulate proliferation, survival, and therapeutic resistance. The overarching goal of the Allen-Petersen research program is to elucidate the role of PP2A in tumorigenesis, therapeutic resistance, and cell plasticity.
Research Area 1: Defining the PP2A-dependent signaling pathways that contribute to cellular plasticity during tumor initiation
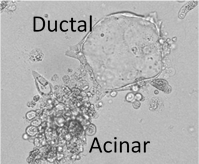
Expression of oncogenes, such as KRAS, can induce the transdifferentiation of pancreatic acinar cells to a duct-like state in a process called acinar-to-ductal metaplasia (ADM). ADM is dependent on transcriptional gene programs that induce dedifferentiation and support a ductal cell fate, while simultaneously suppressing an acinar cell fate. Importantly, the cellular rewiring that occurs during these early tumor initiating events has been shown to have a significant impact on disease progression and therapeutic resistance. Given that PP2A suppresses several key oncogenic pathways implicated in ADM, we aim to define the mechanisms that regulate PP2A during this process, as well as the contribution of this phosphatase to cellular plasticity. We have demonstrated that PP2A suppression leads to epigenetic rewiring and increases the propensity of pancreatic acinar cells to transition to a duct-like fate in response to mutant KRAS expression. We are now defining the direct PP2A targets
Research Area 2: Determine the impact of PP2A dysregulation on tumor progression and metabolic plasticity
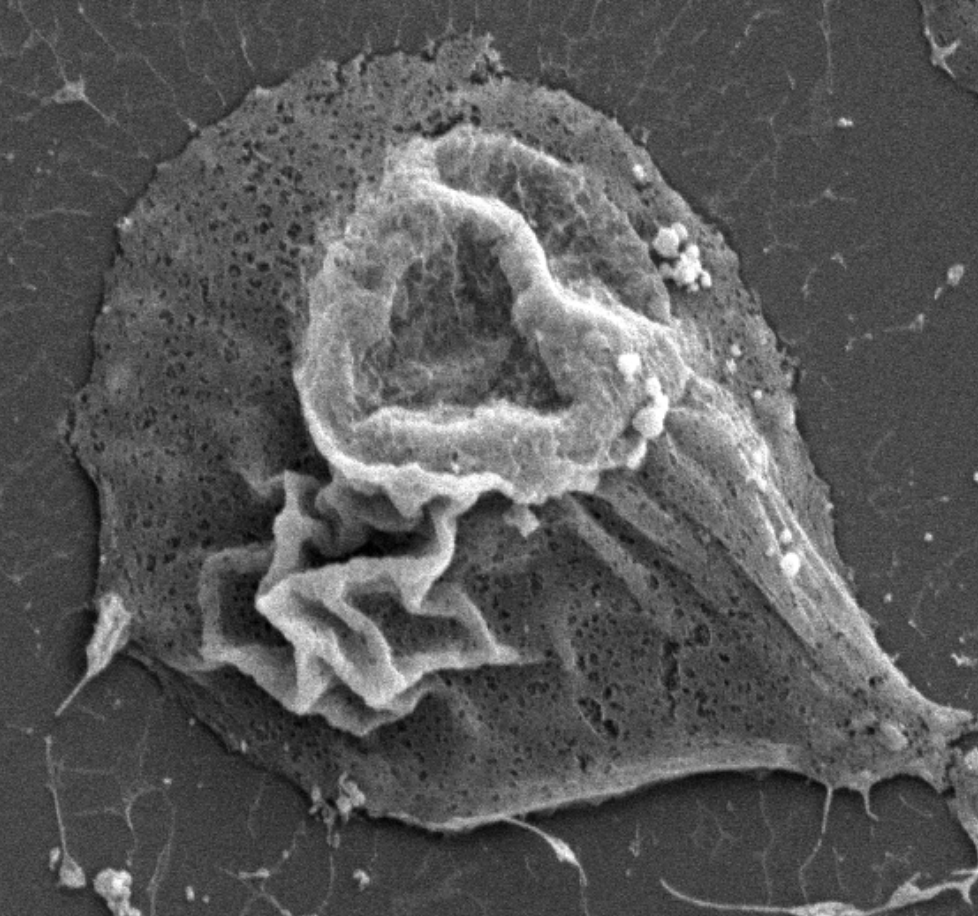
Pancreatic ductal adenocarinoma (PDAC) cells undergo extensive metabolic reprogramming to sustain uncontrolled growth. During times of nutrient stress, PDAC cells take advantage of an evolutionarily conserved nutrient scavenging process called, macropinocytosis, where cancer cells indiscriminately engulf large amounts of extracellular fluid. The process of macropinocytosis is driven by phosphorylation cascades downstream of growth factor receptors and KRAS signaling. However, the regulation of this process by protein phosphatases is unknown. Therefore, the Allen-Petersen lab aims to determine the role of PP2A in the regulation of macropinocytosis, taking advantage of novel mouse models and small molecule activators of PP2A. Given the unique utilization of macropinocytosis for nutrient acquisition, inhibition of macropinocytosis represents a novel therapeutic strategy for PDAC patients.
In our efforts to uncover the functional role of specific PP2A subunits to PDAC progression, the Allen-Petersen lab paradoxically identified that a subset of PDAC cells exhibited reduced tumorigenic properties in response to PP2A suppression, while overexpression of PP2A exacerbated these phenotypes. These findings directly contradict established tumor suppressor roles for PP2A. We have now established that activation of PP2A elicits an oncogenic feedback loop through EGFR signaling pathways that dampens the tumor suppressive functions of PP2A. Currently, we are interrogating the unique signaling pathways that underlie these phenotypes and the therapeutic potential of PP2A activators combined with EGFR inhibitors. These studies suggest that PP2A function is highly context dependent and underscore a clinical need for increased understanding of PP2A biology in cancer.
Research Area 3: Identify the posttranslational mechanisms by which PP2A regulates epithelial to mesenchymal transition and metastasis
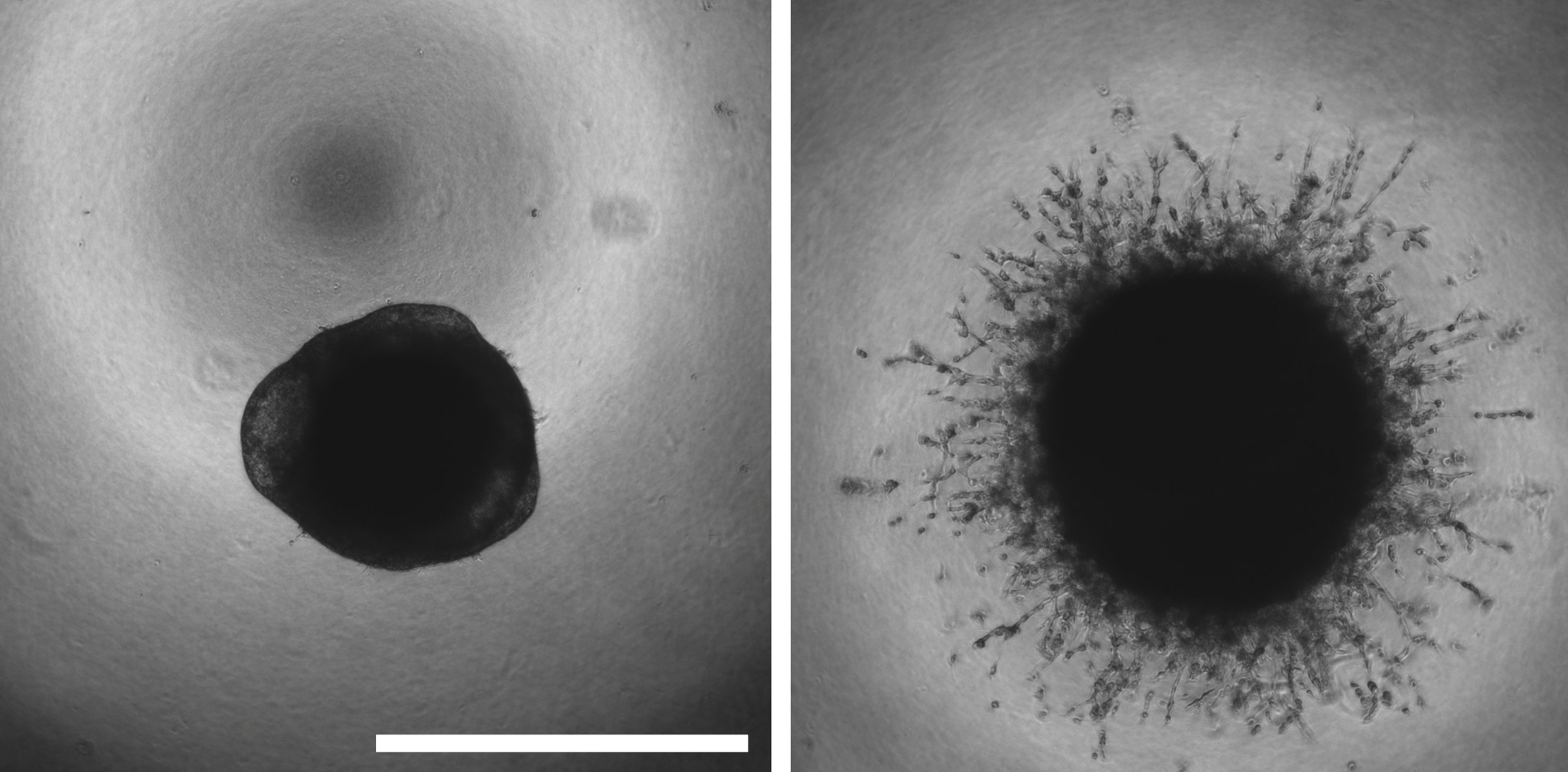
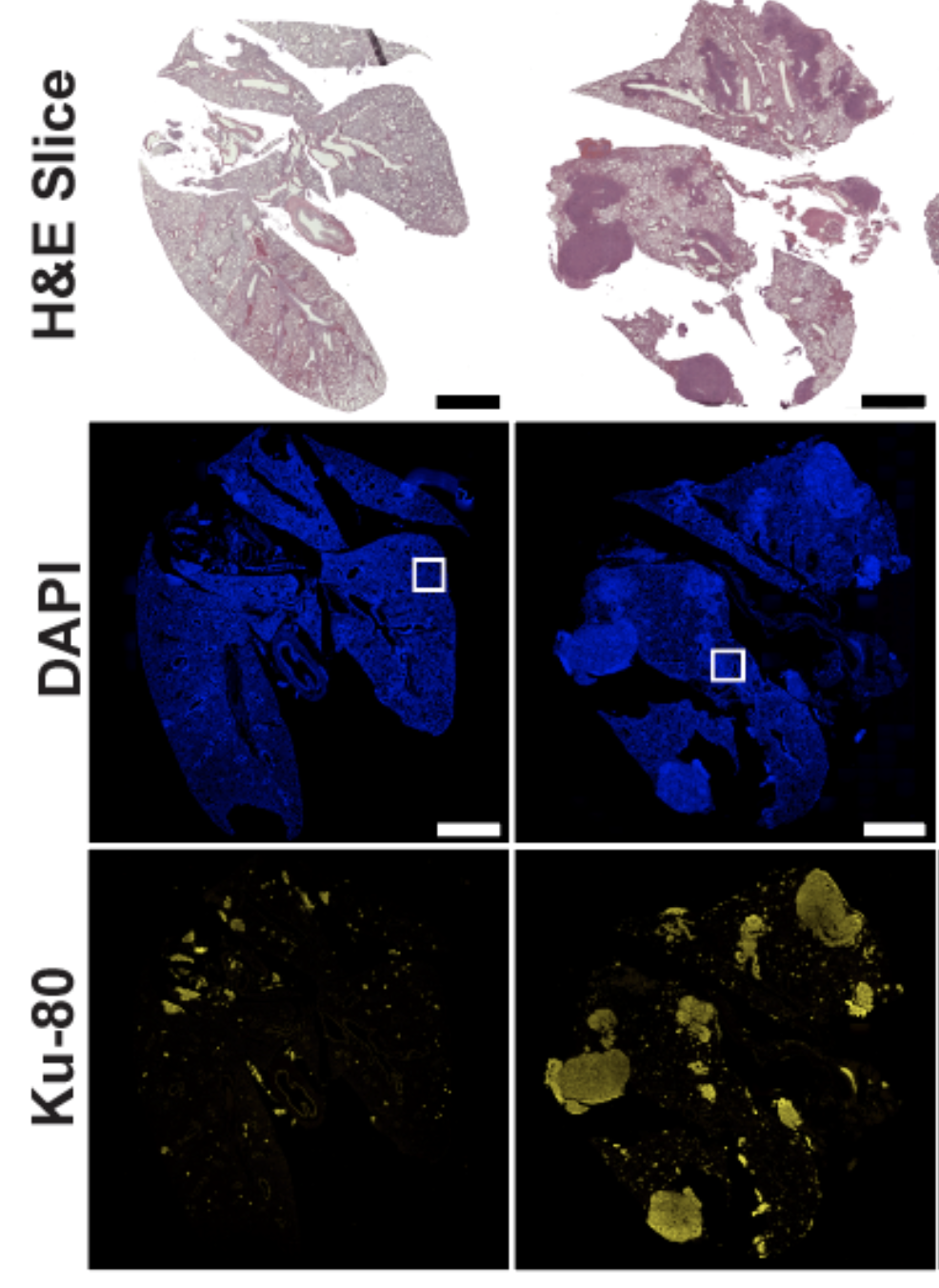 Epithelial-to-mesenchymal transition (EMT) is a dynamic form of cellular plasticity that is associated with metastatic potential, tumor heterogeneity, and drug resistance. During EMT, cancer cells suppress the expression of epithelial markers,decreasing cell-cell junctions, and increase mesenchymal markers that drive invasion and metastatic dissemination. Given that metastasis is the major causeof cancer-related deaths, the therapeutic targeting of this reprogramming represents a significant strategy to improve patient outcomes. PP2A has been implicated in several steps of the metastatic cascade, and overexpression of the PP2A catalytic subunit has been shown to suppress EMT phenotypes. Together, these studies broadly implicate PP2A in the direct negative regulation of EMT programs during cancer dissemination; however, the subunits required for this regulation and the mechanism by which PP2A suppresses EMT is unknown. The Allen-Petersen lab has discovered the suppression of the PP2A B subunit, B56a, potently drives EMT in a subset of pancreatic and lung cancer cell lines resulting in a significant increase in metastatic potential both in vitro and in vivo. These phenotypic changes are associated with large-scale phosphoproteomic changes consistent with dedifferentiation and drug resistant cell states. Furthermore, overexpression of B56a is able to reverse these phenotypes, highlighting the highly dynamic and transient nature of these changes.
Epithelial-to-mesenchymal transition (EMT) is a dynamic form of cellular plasticity that is associated with metastatic potential, tumor heterogeneity, and drug resistance. During EMT, cancer cells suppress the expression of epithelial markers,decreasing cell-cell junctions, and increase mesenchymal markers that drive invasion and metastatic dissemination. Given that metastasis is the major causeof cancer-related deaths, the therapeutic targeting of this reprogramming represents a significant strategy to improve patient outcomes. PP2A has been implicated in several steps of the metastatic cascade, and overexpression of the PP2A catalytic subunit has been shown to suppress EMT phenotypes. Together, these studies broadly implicate PP2A in the direct negative regulation of EMT programs during cancer dissemination; however, the subunits required for this regulation and the mechanism by which PP2A suppresses EMT is unknown. The Allen-Petersen lab has discovered the suppression of the PP2A B subunit, B56a, potently drives EMT in a subset of pancreatic and lung cancer cell lines resulting in a significant increase in metastatic potential both in vitro and in vivo. These phenotypic changes are associated with large-scale phosphoproteomic changes consistent with dedifferentiation and drug resistant cell states. Furthermore, overexpression of B56a is able to reverse these phenotypes, highlighting the highly dynamic and transient nature of these changes.