Projects
The transport and life cycle of mitochondria in the nervous system.
|
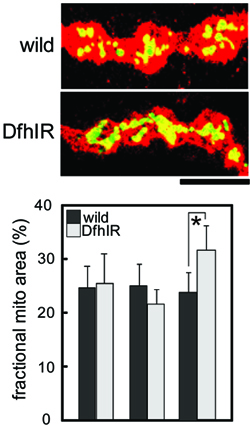
We are trying to understand how the transport of mitochondria over long distances in axons is coordinated with their major physiological functions and with other aspects of their life cycle. We can quantify their movements toward and away for the neuronal cell bodies, the resulting steady-state distribution and density in axons, and their membrane potential, ROS production and morphology. We also study their fission, fusion and turnover by autophagic sequestration. In vertebrate systems we have studied how they coordinate with axonal branching. All these features of the mitochondrial life cycle involve complex, coordinated events, and defects in any or all of them would result in pathology. Thus, we are testing whether and exactly how the mitochondrial life cycle is disrupted in fly models of human neurodegenerative diseases such as Friedreich ataxia and Parkinson’s disease. Shidara, Y. & P. J. Hollenbeck. 2010. Defects in mitochondrial axonal transport and membrane potential without increased ROS production in a Drosophila model of Friedreich ataxia. Journal of Neuroscience 30:11369-11378. Ruthel, G. & P. J. Hollenbeck. 2003. Response of mitochondrial traffic to axon determination and differential branch growth. Journal of Neuroscience 23:8618-8624. |
 |
Control of mitochondrial transport and function by cell signaling.
|
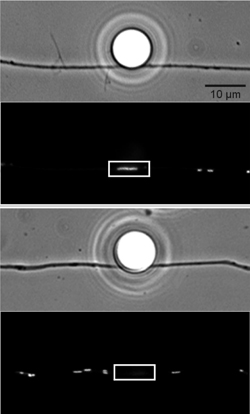
Although mitochondria have considerable autonomy relative to other organelles, their function is nonetheless intimately tied to the rest of the cell. We have studied the role of specific cell signaling pathways in the transport and positioning of mitochondria in axons. We have found that nerve growth factor signaling can attract and dock mitochondria to actin in specific regions of the axon, and that this effect is mediated in part via the PI3 kinase pathway. We are currently assessing the role of ROS signaling in mitochondrial motility and metabolic function. Pathak, D., Sepp, K. & P. J. Hollenbeck. 2010. Evidence that myosin opposes microtubule-based axonal transport of mitochondria. Journal of Neuroscience 30:8984-8992. Verburg, J. L. & P. J. Hollenbeck. 2008. Mitochondrial membrane potential in axons increases with local NGF or semaphorin signaling. Journal of Neuroscience 28:8306-8315. Amiri, M. & P. J. Hollenbeck. 2008. Mitochondrial biogenesis in the axons of vertebrate peripheral neurons. Developmental Neurobiology 68:1348-1361. Chada, S. R. and P. J. Hollenbeck. 2004. Nerve growth factor signaling regulates motility and docking of axonal mitochondria. Current Biology 14:1272-1276. Chada, S. and P. J. Hollenbeck. 2003. Mitochondrial movement and positioning in axons: the role of growth factor signaling. Journal of Experimental Biology 206:1985-1992.
|
 |
Response of mitochondria to infection of cells by vacuole-forming bacteria.
|
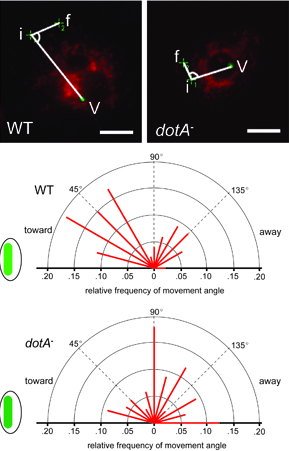
When bacteria such as Legionella infect cells, they inject bacterial proteins into the cytoplasm that hijack many cellular processes and make the vacuole and the cell favorable sites for bacteria to replicate. The response of mitochondria to cellular infection has been described anecdotally but neither demonstrated directly and quantitatively nor explained mechanistically. We have studied mitochondrial behavior and function rigorously in various cell types and find that there are cells, such as Drosophila S2 cells, that can harbor productive Legionella infection without any effect on mitochondrial behavior. Other cells, such as physiologically relevant host macrophages, do show pronounced mitochondrial responses to infection. We are investigating whether and how Legionella infection affects mitochondrial movement, distribution, metabolism and ROS production in host cells, and how this contributes to infection or immunity. Sun, E.W., Wagner, M. L., Maize, A., Kemler, D., Garland-Kuntz, E. Xu, L., Luo, Z.Q. and P. J. Hollenbeck. 2013. Legionella pneumophila infection of Drosophila S2 cells induces only minor changes in mitochondrial dynamics. PloS ONE 8(4): e62972. |
 |

