E. Technical Reports
Systems Analysis of the Dynamics of Membrane Architecture, Composition, and Function
Himadri Pakrasi, Michelle Liberton, Jana Stockel, and Eric Welsh
Cyanothece sp ATCC 51142 is a metabolically versatile organism, with an intriguing ability to store photosynthetically fixed carbon in glycogen granules during the day, and utilize these stored carbons for biosynthesis and energy production during night. Our overall goal is to use systems-level approaches to develop a detailed and predictive physiological model for this bacterium. Specifically, the activities in our research group have been focused on three areas: 1) sequencing and annotation of the genome of Cyanothece 51142, 2) transcriptomic and proteomic analyses of the diurnal rhythm in Cyanothece 51142, and 3) ultrastructural studies of Cyanothece cell membranes during a diurnal cycle.
Sequencing and Annotation of the Genome of Cyanothece 51142
Cyanothece 51142 is a unicellular diazotrophic marine cyanobacterium capable of separating the incompatible processes of oxygenic photosynthesis and nitrogen fixation temporally within the same cell, performing 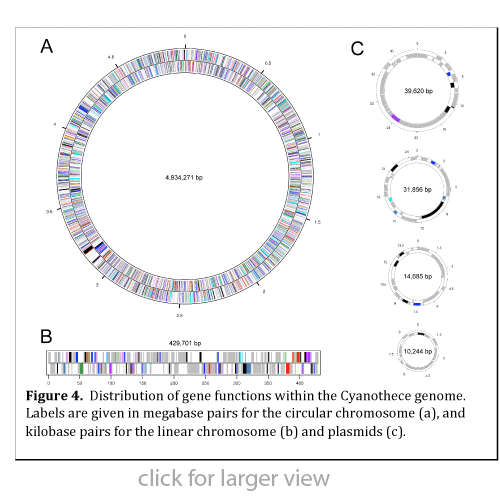 photosynthesis during the day and nitrogen fixation at night. The genome of Cyanothece 51142 was sequenced and found to contain a unique arrangement of one large circular chromosome, four small plasmids, and one linear chromosome, the first report of such a linear element in a photosynthetic bacterium (see Figure 4). Annotation of the Cyanothece genome was aided by the use of high-throughput proteomics data, enabling the reclassification of nearly 25% of the predicted hypothetical proteins. Phylogenetic analysis suggests that nitrogen fixation is an ancient process that arose early in evolution and has subsequently been lost in many cyanobacterial strains.
photosynthesis during the day and nitrogen fixation at night. The genome of Cyanothece 51142 was sequenced and found to contain a unique arrangement of one large circular chromosome, four small plasmids, and one linear chromosome, the first report of such a linear element in a photosynthetic bacterium (see Figure 4). Annotation of the Cyanothece genome was aided by the use of high-throughput proteomics data, enabling the reclassification of nearly 25% of the predicted hypothetical proteins. Phylogenetic analysis suggests that nitrogen fixation is an ancient process that arose early in evolution and has subsequently been lost in many cyanobacterial strains.
Transcriptomic and proteomic analyses of the diurnal rhythm in Cyanothece 51142
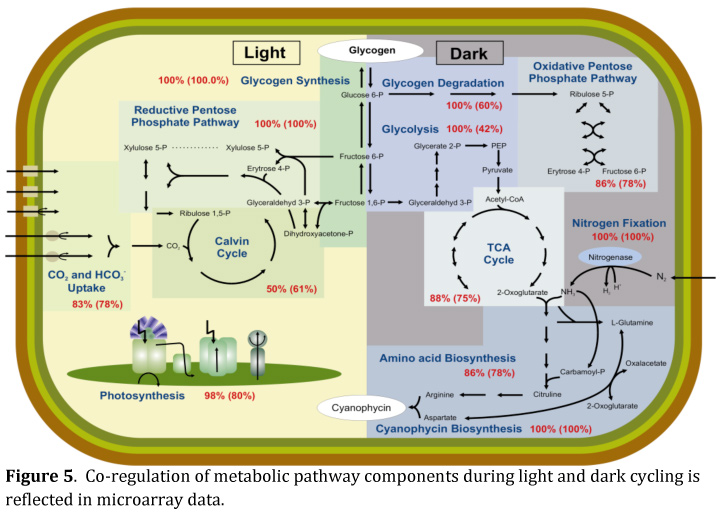
Unicellular diazotrophic cyanobacteria, such as Cyanothece 51142, are challenged with creating and maintaining both aerobic and obligate anaerobic intracellular conditions to fulfill the requirements for oxygenic photosynthesis and nitrogen fixation during a diurnal cycle. Key to deciphering the cellular processes involved in regulating such a dramatic cycle is the identification of gene regulatory events. Global gene expression analysis in Cyanothece pinpointed a significant impact of nitrogen fixation on the diurnal cycle of different fundamental pathways and thus provided new systems-level insights. Our study revealed that 27% of all genes in the genome exhibit oscillating expression patterns. Among those genes, 48% are of unknown functions. We observed a remarkable co-regulation of functionally related genes and entire pathways coupled with an increased metabolic diversity and activity during the night (see Figure 5). Furthermore, we found that steady state transcript abundance cycles with a significantly higher transcript accumulation during the dark period. Our analyses suggest that nitrogen fixation, not photosynthesis is determining the major metabolic activities inside the cell and is thus driving the majority of cellular activities
Parallel to the transcriptomic analysis, we collected samples for global proteomic analysis of Cyanothece during a diurnal cycle. These samples were sent to Jon Jacobs and his colleagues at PNNL. Detailed analysis of these samples is in progress and included in the Proteomics’ team report.
Ultrastructural studies of Cyanothece cell membranes during a diurnal cycle
We have recently completed a detailed tomographic analysis of the membrane architecture in Cyanothece 51142 cells. Cyanobacterial cells in general, and Cyanothece 51142 in particular, have a typical gram-negative envelope layer. In addition, they have an elaborate internal thylakoid membrane system. Cyanobacteria are the progenitors of plant chloroplasts. Thylakoids in cyanobacteria and the chloroplasts of algae and plants are the sites where photosynthetic electron transport occurs, an enzymatic reaction fundamental to planetary life. The morphology of thylakoid membranes is intimately related to the physiology of the reactions that occur there, but many details of thylakoid morphology are not well understood, particularly in plant chloroplasts. Cyanobacteria, as the progenitors of chloroplasts, provide a model system to explore the origin of thylakoid morphology. In Cyanothece sp. ATCC 51142, a unicellular cyanobacterium, thylakoids display a rudimentary helical organization, a potential evolutionary step to the modern grana and stroma thylakoid arrangement in plant chloroplasts. Our studies have indicated that a defining feature of chloroplast thylakoids is present in a cyanobacterium, and is not a new invention in plants.
Analyses of the Unicellular, Diazotrophic Cyanobacterium Cyanothece sp ATCC 51142: Genetics, Imaging and Metabolic Rhythms during Growth in a 6-L Bioreactor
Louis Sherman, Hongtao Min, and Jörg Toepel
The main goals of this project are to study Cyanothece throughout its diurnal cycle from many different perspectives and to obtain the annotated genome. The ultimate objective is to develop a complete metabolic and structural model to determine how cellular components, especially membranes, operate as a function of time throughout the day/night cycle. Our component of the overall program involves three projects. The first is genetics, so that we can target specific genes in order to knockout their ability to produce their protein products. In this way, we can test very precisely the type of predictions made in the overall metabolic model. A second objective has been to use the modern techniques of high-pressure freezing and 3-D tomography in order to determine the precise structure of the cell and cellular components. The third objective has been to determine appropriate conditions for growth of Cyanothece in a large bioreactor such that similar growth conditions can be achieved at Purdue, Washington University, and PNNL.
The basic premise for the project was established in this lab some years ago. At that time, we determined that this cyanobacterium could grow beautifully under N2-fixing conditions and that it separated the oxygen-sensitive nitrogenase from oxygen evolving photosynthesis via temporal regulation. Thus, when cells were grown under N2-fixing conditions, nitrogen fixation took place exclusively at night and photosynthesis exclusively in the dark. However, the metabolic rhythms persisted for many days and it was clear that the entire process was controlled by the circadian clock. At that time, all of the work was done one gene or one protein at a time and, although we developed an overall idea of the process, our overall knowledge was rather limited. Therefore, the ability to study virtually all components of the cell using the more modern high throughput techniques was both daunting and exhilarating. Therefore, one major objective of this project has been to obtain transcriptomics, proteomics and metabolomics data as a function of time over the daily life cycle of Cyanothece. Our involvement has been to lead the research in areas such as genetics and cultivation and then to provide material to various groups at EMSL for analysis by a variety of techniques. We have performed the transcriptomics ourselves using Agilent microarrays developed by Rajeev Aurora (see his technical report for more information). This project demands the talents and techniques of many different types of scientists and can only be successful in collaboration with scientists at EMSL.
GeneticsWe quickly worked out procedures for transformation using techniques such as electroporation and CaCl2 treatment. We used a broad-host-range plasmid, pRL1383a, and obtained high frequencies of transformation depending upon the exact nature of growth. The efficiency of transformation depended upon growth conditions and, the faster the growth, the higher the efficiency of transformation. We then determined that the same growth rate dependency was true for a fraction of DNA that we first thought was plasmid. This is where the genome sequence data became very important. The indication that Cyanothece contained a linear chromosome provided an important clue to our situation. Thus, we hypothesized that the higher growth rates generated a higher copy number of the linear chromosome and led to a higher rate of transformation. We have shown that this correlation is correct.
Our second goal in genetics was homologous recombination that would be used to construct individual gene knockout mutants so that we could target specific genes for mutagenesis. However, we have not yet been able to construct such knockouts, in part, because there is both non-homologous and site-specific recombination occurring in Cyanothece that competes with the homologous recombination. From the sequence, we know that there is a site specific recombinase that targets the DNA into specific sites and overwhelms the targeted mutagenesis into specific genes. We are now developing approaches that can overcome this problem. However, the genome sequences also provide us with some very valuable information so that we may be able to use an RNAi-like system to target specific genes and to produce what is called knock-down mutations. We have also begun introducing GFP markers into strains of Cyanothece.
Imaging of Dynamic Membrane StructuresOne objective has been to identify the best procedures for the study of the 3-dimensional structure of Cyanothece. We have worked with high pressure freeze (HPF) techniques and freeze substitution to develop fixation procedures that stabilize membranes and internal storage granules. Meeting both goals was difficult, but we have done so and provided this information to other labs within the project. We have now used these procedures to obtain high-resolution tomographic images of Cyanothece and are working to develop a model of glycogen granule structure in relation to the photosynthetic membrane. We are also interested in the relationship of the photosynthetic and cytoplasmic membranes and other particles that interact with one or both membrane systems. We have found that the glycogen granules are not necessarily discreet entities, but represent beads on a string (see Figure 6). Therefore, depending upon the projection, the granules come together and all the glycogen granules between two photosynthetic membranes are in contact with each other.
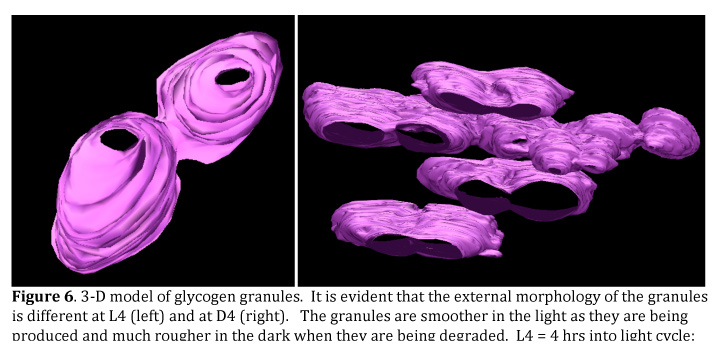
These images have also given us a good idea of how the glycogen granules form or are degraded in the light and dark, respectively. We have also imaged a number of small particles near the interface of the photosynthetic and cytoplasmic membranes and we are interested to know if these are involved in assembly. We are now completing the modeling of these structures so that we have a good representation of these components within a part of the cell.
Cultivation and Regulation of Cell Processes during Diurnal Cycling
One of our objectives was to develop a bioreactor suitable for consistent growth of a cyanobacterium-like Cyanothece. The bioreactor was developed in conjunction with Dr. Pakrasi and Dr. Gorby and has proven to be well conceived. The resulting vessel is good for cell growth and for metabolic analyses and provides us with the type of information we need to investigate the metabolic rhythms. We have found that cells can be grown with a high degree of reproducibility under nitrogen-fixing, light-dark conditions including with periods of continuous light or continuous dark. Such cells are very well synchronized and can be used for all of the high throughput experiments discussed above.
We have concentrated specifically on physiological and transcriptional analysis of cells growing under light-dark conditions with periods of continuous light. The specific question that we have asked is: how does the regulation of cells growing under a subjective dark condition (in the presence of light) compare to true dark? We have used a variety of different primers, including pH and dissolved oxygen, which can be measured in the bioreactor, along with photosynthesis, respiration, nitrogen fixation and glycogen concentration measurements. We determined that glycogen is not degraded as quickly in continuous light and that glycogen storage remains high under these conditions (see Figure 7). Once cells are returned to the dark, there is a huge N2-fixation peak; in addition, respiration is very high and glycogen is quickly degraded. We have done a thorough transcriptional analysis of both light/dark and continuous light, that follows on the work done by Jana Stockel in Dr. Pakrasi’s laboratory. We were able to show that our results compare very nicely to those done under light/dark conditions at Washington University, and we also obtained
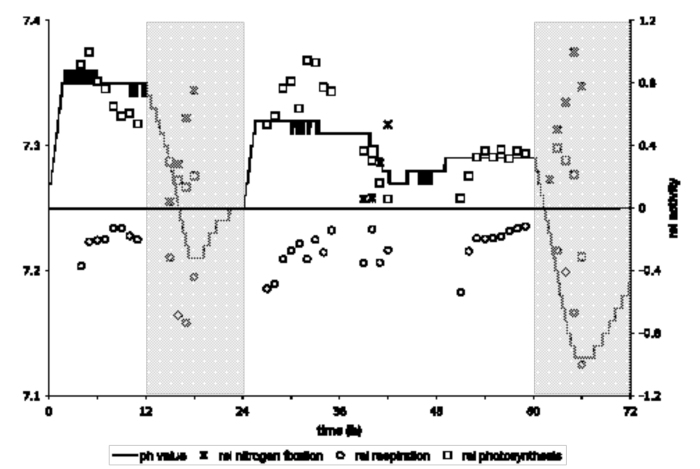
Figure 7. Cyanothece response to a light-dark to light-light to light-dark transfer in the absence of nitrogen. Cyanothece showed the highest nitrogen fixation activity at D6, combined with high respiration rate and a reduced photosynthetic rate. Under continuous light, the nitrogen fixing activity was reduced by 50% and the respiration was not enhanced, but both processes still peaked at D6.
significant data on the transcription during continuous light (LL) versus light/dark cycling (LD). In particular, we found that the gene encoding the protein involved in glycogen degradation is not induced as much as in the subjective dark (second light phase of LL) as in the regular dark providing a reason for the high accumulation of glycogen in the subjective dark. We are now in the process of providing such material to the metabolomics and proteomics group to obtain a thorough understanding of these phenomena.
