Research
Rapid drug discovery for retinal degeneration
We try to speed up eye-drug discovery by studying the zebrafish model. It is a highly visual animal. Its visual responses have been used to identify eye-disease mutants. For example, many retinal-degeneration mutants lack an optokinetic response (OKR).
The OKR of a retinal-degeneration mutant (top) and its normal sibling (bottom). These larvae are put inside a rotating drum with black and white stripes (the reflection of the pattern can be seen in the air bubble on the left). Even though both larvae look normal, only the bottom one can track the rotating stripes and move its eyes accordingly.
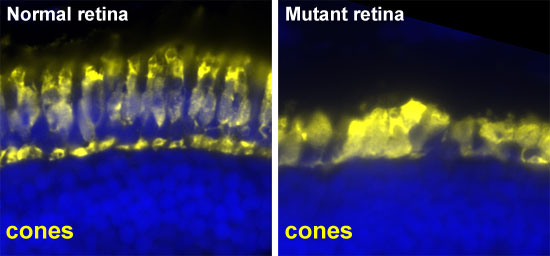 Histological analysis of the retinas of these larvae. The cone photoreceptors are labeled in yellow. These photoreceptors are degenerating in the mutant but not in the normal sibling. This affects the vision of the mutant and results in no response to the OKR test.
Histological analysis of the retinas of these larvae. The cone photoreceptors are labeled in yellow. These photoreceptors are degenerating in the mutant but not in the normal sibling. This affects the vision of the mutant and results in no response to the OKR test.
Since the little zebrafish embryos can absorb chemicals through their skin, they can absorb drugs we put in the water. Then, we can efficiently evaluate the potential therapeutic value of the drugs by measuring the effects of drug treatment on the light sensation and associated histology of the zebrafish. Our major screening platform, the visual-motor response (VMR) assay, involves simultaneous testing of the light sensation of multiple zebrafish embryos in multi-well plates.
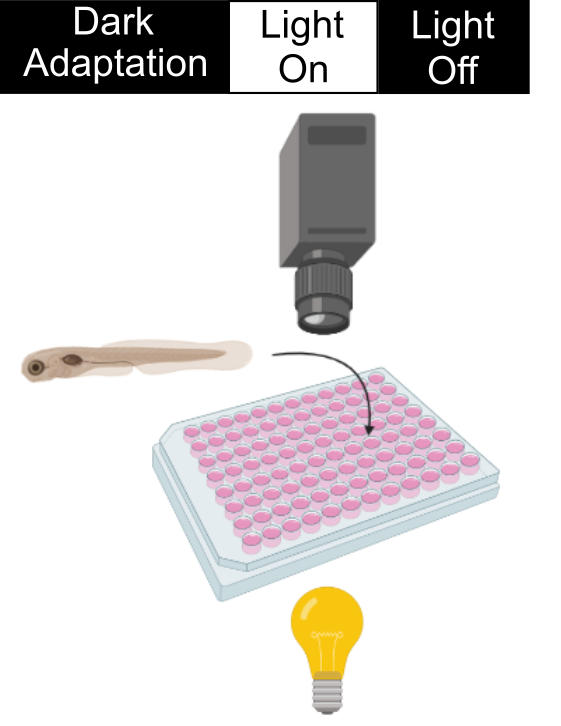 During a VMR experiment, we put the 96-well plate with zebrafish embryos in a dark machine to allow them to get used to the environment. Then we will stimulate them with a sudden light onset and offset. These embryos naturally do not like a drastic change in light illumination and will swim uniquely during light changes. These swimming patterns are captured by the camera and analyzed by a computer. In the retinal-degeneration models, their photoreceptors degenerate. Consequently, they cannot detect the light change as well as the healthy zebrafish (See an example below). In our drug screen, we put drugs in the wells of the retinal-degeneration mutants. Those drugs that increase the VMR of the retinal-degeneration mutants are good candidates for treating the condition. An example will be given below.
During a VMR experiment, we put the 96-well plate with zebrafish embryos in a dark machine to allow them to get used to the environment. Then we will stimulate them with a sudden light onset and offset. These embryos naturally do not like a drastic change in light illumination and will swim uniquely during light changes. These swimming patterns are captured by the camera and analyzed by a computer. In the retinal-degeneration models, their photoreceptors degenerate. Consequently, they cannot detect the light change as well as the healthy zebrafish (See an example below). In our drug screen, we put drugs in the wells of the retinal-degeneration mutants. Those drugs that increase the VMR of the retinal-degeneration mutants are good candidates for treating the condition. An example will be given below.
 Measuring the VMR of 96 zebrafish in a 96-well plate.
Measuring the VMR of 96 zebrafish in a 96-well plate.
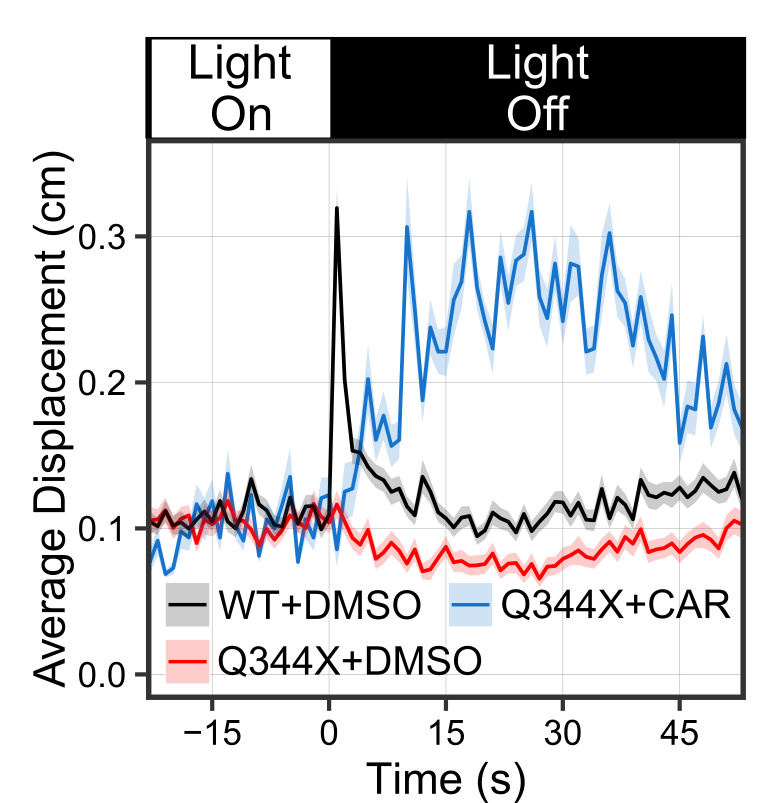 CAR treatment increased the VMR of the Q344X adRP mutant. These graphs show the average VMR displayed by the healthy embryos (wildtype/WT) or Q344X mutant. They were treated either with CAR or a drug carrier/solvent called DMSO as treatment control. Without CAR treatment, the WT+DMSO (black curve) displayed a typical light-offset VMR, whereas the Q344X+DMSO (red curve) displayed a reduced VMR due to rod degeneration (also see next picture). With CAR treatment, however, the Q344X+CAR (blue curve) displayed an enhanced VMR compared with the Q344X+DMSO. As it turns out, these treated mutant embryos got more rod photoreceptors.
CAR treatment increased the VMR of the Q344X adRP mutant. These graphs show the average VMR displayed by the healthy embryos (wildtype/WT) or Q344X mutant. They were treated either with CAR or a drug carrier/solvent called DMSO as treatment control. Without CAR treatment, the WT+DMSO (black curve) displayed a typical light-offset VMR, whereas the Q344X+DMSO (red curve) displayed a reduced VMR due to rod degeneration (also see next picture). With CAR treatment, however, the Q344X+CAR (blue curve) displayed an enhanced VMR compared with the Q344X+DMSO. As it turns out, these treated mutant embryos got more rod photoreceptors. 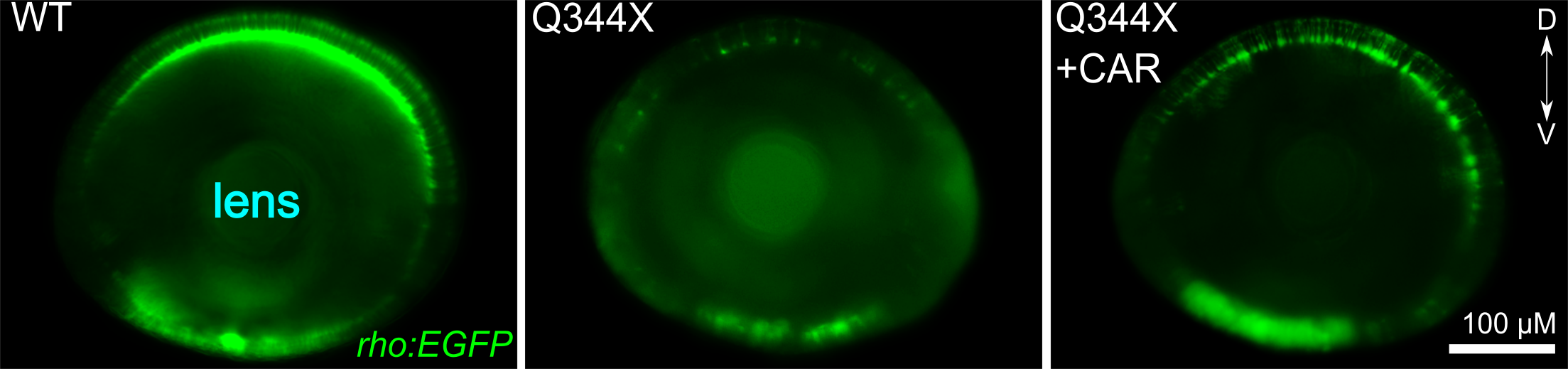 CAR treatment increased rod photoreceptors in the Q344X mutant. These pictures are representative eyeballs dissected from the zebrafish embryo. We are looking at the lens of the eyes and the dorsal side is to the top. Rods are labeled by a green fluorescent protein (EGFP). In WT, we see a lot of rods from this view, especially on the dorsal and ventral sides. In the Q344X mutant, these rods largely degenerate. Our identified drug CAR increased the number of rods in the Q344X mutant, suggesting that this drug may treat the condition.
CAR treatment increased rod photoreceptors in the Q344X mutant. These pictures are representative eyeballs dissected from the zebrafish embryo. We are looking at the lens of the eyes and the dorsal side is to the top. Rods are labeled by a green fluorescent protein (EGFP). In WT, we see a lot of rods from this view, especially on the dorsal and ventral sides. In the Q344X mutant, these rods largely degenerate. Our identified drug CAR increased the number of rods in the Q344X mutant, suggesting that this drug may treat the condition. We have outlined our vision for screening eye drugs with zebrafish in two reviews (Zhang et al., 2012; Ganzen et al., 2017). Together with our other research approach on "elucidating gene network", we can effectively deduce the molecular pathways through which drug candidates exert their therapeutic effects. These endeavors will lay down firm foundations for developing the identified candidate drugs as new treatments for retinal-degenerative diseases.









