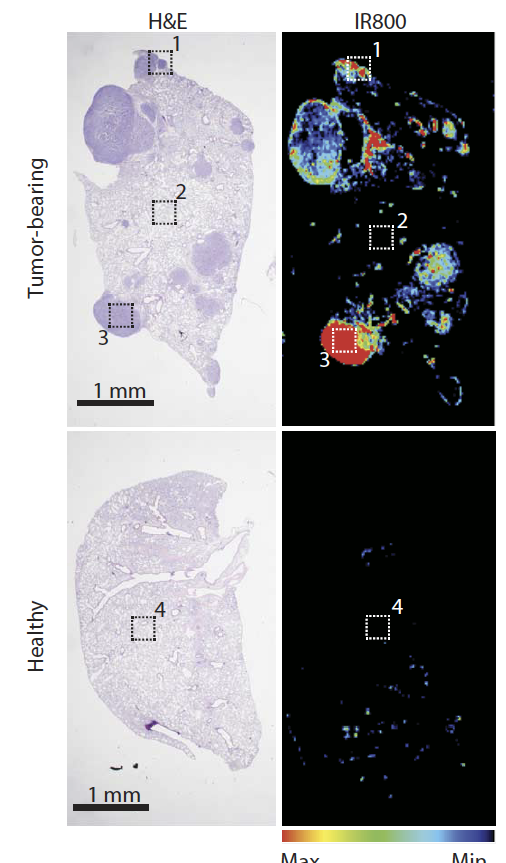
Figure 1: Histological sections of tumor-bearing (top) or healthy (bottom) lungs following administration of folate conjugated to a fluorescent molecule to highlight specificity of folate-conjugate uptake into lung tumors.
Figure 2: Representative MRI images and reconstructed 3D tumor volumes following folate-control (left) or folate-miR-34a delivery. Tumor burden in folate-miR-34a treated animals was significantly reduced.
Project 2:
Ligand-mediated delivery of small RNAs
One of the biggest hurdles that we face when advancing RNAs into the clinic is delivery. Getting RNAs to the correct cells, in the absence of degradation and toxicity has been a challenge in the field. Overcoming this major obstacle requires an approach that i) delivers RNAs rapidly, to avoid degradation in circulation, ii) delivers the RNAs selectively, to avoid non-diseased cells, and iii) a mechanism that alleviates toxicity that is often associated with the delivery vehicle.
Recently, we pioneered a novel and highly effective way of administering small RNAs in vivo that achieves each of these requirements. This method relies on conjugating the small RNA directly to a ligand whose receptor is overexpressed on tumor cells, in this case folate and the folate receptor, respectively. These folate-miRNA conjugates, which we have termed FolamiRs are taken up rapidly and specifically by both breast and lung tumors in vivo. When a tumor-suppressive miRNA, in this case miR-34a, is delivered using this method tumor growth was significantly diminished. Importantly, in a maximum tolerated dose study, we were unable to identify a dose that was intolerable; no signs of toxicity were observed even following the administration of doses 33-fold higher than the efficacious dose.
This area of research is being expanded to other targeting ligands and receptors, such as DUPA:PMSA for prostate cancer. We are also working to further reduce dosing by way of enhancing endosomal escape of the ligand conjugates.