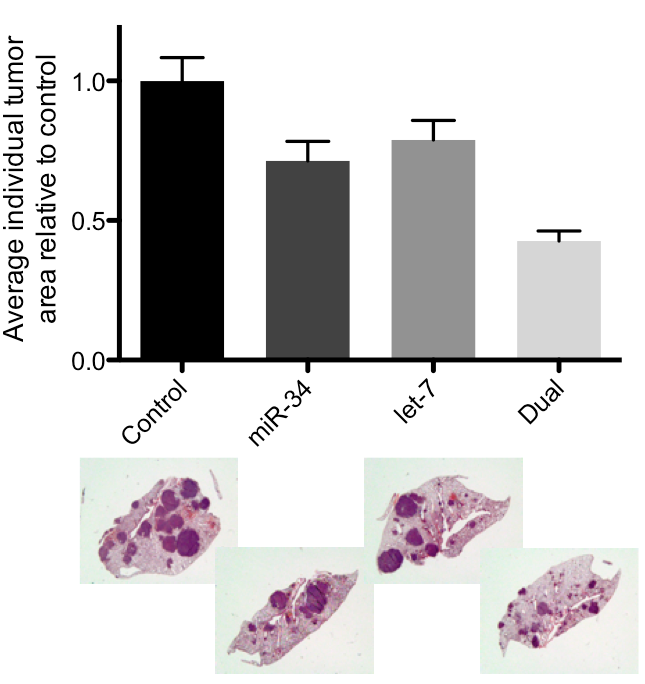
Figure 1: Kras;p53 tumor-bearing mice were treated systemically with the indicated miRNAs encapsulated in a lipid-based delivery vehicle. Animals treated with the combination showed a marked reduction in tumor burden and average tumor area.
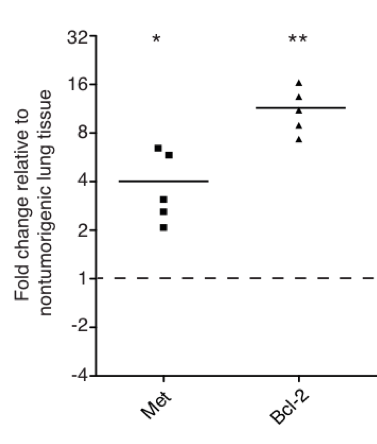
Figure 2: Quantitative RT-PCR for two miR-34 target genes from Kras;p53 tumors. Both Met and Bcl-2 are elevated in the lungs of tumor-bearing mice.
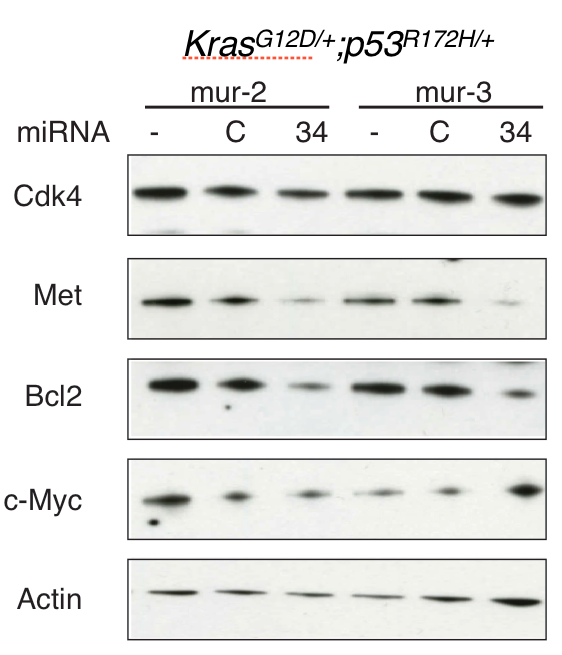
Figure 3: Downregulation of Bcl-2 and Met following ectopic delivery of miR-34 in Kras;p53cell lines.
Project 1:
Using tumor suppressive miRNAs as cancer therapeutics and sensitizers
There has been ample progress in miRNA-directed therapeutics for some obvious miRNA candidates, such as let-7 and miR-34. let-7 and miR-34 act as tumor suppressors through targeting multiple oncogenes (e.g. RAS, MYC, CCND, BCL2, MET). As such, let-7 and miR-34 can affect multiple cancer pathways with a single drug administration, analogous to a multi-drug cocktail. Since miRNAs are natural gene products (let-7 and miR-34 are expressed in normal differentiated cells, but not in stem cells and cancer stem cells) their use as drugs is not predicted to lead to significant side effects. In fact, in recent studies performed by our lab and others, no toxicities from miRNA delivery have yet been reported. Although small RNA delivery has been viewed as a challenge, both in protecting the RNA drug and delivery of the drug to the right tissues, our current work in collaboration with Dr. Andy Bader (Mirna Therapeutics, Inc.) has overcome this concern with respect to delivery of miRNA mimetics to lung tissue using a lipid based nanoparticle called a Smarticle (Marina Biotech), which is already in human clinical trials (ProZAi Therapeutics, Inc.).
There is compelling evidence that miRNAs such as let-7 and miR-34 are master regulators in the cancer phenotype. Our recent work supports this hypothesis in Kras;p53 mouse models: the let-7/miR-34 combination induces a tumor-static response that is better than either individual miRNA (Figure 1). Further, although Bcl-2 and c-Met are elevated in Kras;p53 tumors (Figure 2), miR-34 reduces both targets (Figure 3), while let-7 represses the Kras oncogene. Tumors fail to progress in animals treated systemically with the combination, resulting in smaller and potentially more manageable tumors (Figure 1). As such, this project is based on the hypothesis that miR-34 and let-7 can sensitize tumors to other therapeutic regiments, such as erlotinib, gefitinib, and cisplatin, common therapies used in the treatment of NSCLC.