-
Glukhova A, Hinkovska-Galcheva V, Kelly R, Abe A, Shayman JA, Tesmer JJ. Structure and function of lysosomal phospholipase A2 and lecithin:cholesterol acyltransferase. Nat Commun. 2015 6:6250. PMC4397983
-
Hoadley-Manthei K, Ahn J, Glukhova A, Yuan W, Larkin C, Manett TD, Chang L, Shayman JA, Axley MJ, Schwendeman A, Shayman JA, Tesmer JJG: A retractable lid in lecithin:cholesterol acyltransferase provides a structural mechanism for activation by apolipoprotein A-I. J. Biol. Chem. 2017 292:20313-20327. PMC5724016
-
Manthei KA, Yang S-M, Baljinnyam B, Glukhova A, Chang L, Yuan W, Freeman LA, Shen M, Maloney DJ, Schwendeman A, Remaley AT, Jadhav A, and Tesmer JJG: Molecular basis for activation of lecithin:cholesterol acyltransferase (LCAT) by a high affinity chemical probe. Elife, 2018 7: pii: e41604. doi: 10.7554/eLife.41604. PMC6277198
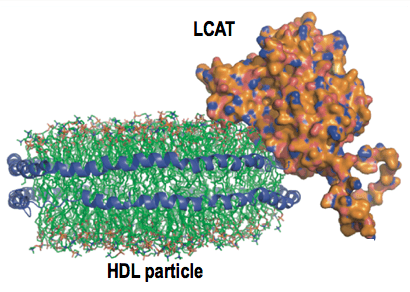
- Manthei KA, Patra D, Wilson CJ, Fawaz MV, Piersimoni L, Shenkar J, Yuan W, Andrews PC, Engen JR, Schwendeman A, Ohi MD, Tesmer JJG. Structure of lecithin:cholesterol acyltransferase bound to high density lipoprotein particles. Commun. Biol. 2020, 3:28. doi: 10.1038/s42003-019-0749-z. PMC6962161